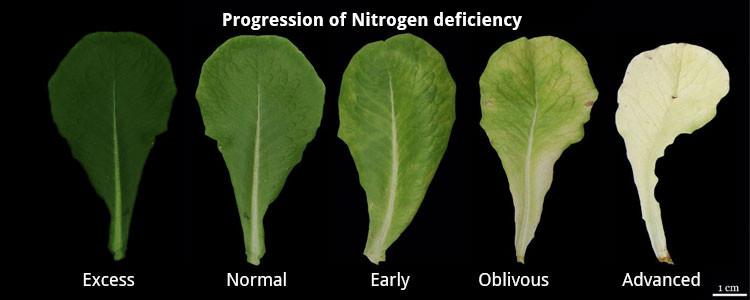
|
Nutrients, the environment, plant leaves, and photosynthesis
Nutrients, chemical compounds or ions in the environment, are necessary for plant survival, growth and reproduction. Plants must photosynthesize to survive; photosynthesis is the passive uptake of carbon-dioxide and water for the autotropic production of carbohydrates using sunlight. Therefore, plants require water, carbon-dioxide and sunlight. Carbon (C), Hydrogen (H), and Oxygen (O) an all be readily obtained from the air and water. Over three quarters of the air is Nitrogen (N), in the form of di-nitrogen gas, but this form is inaccessible to plants. Other nutrients must come from the soil, and therefore the performance of plants is directly related to the degree of fertility of the soil they occupy. Phosphorus (P), N, and Potassium (K), are the three principle macronutrients that together or separately can limit plant performance. N is needed for photosynthetic activity in the leaves, P promotes root development and K is used throughout the plant, and in conjunction with N usually, in photosynthesis and to promote overall plant health, like protecting against temperature extremes or defending against disease. Several other micronutrients are necessary for plant growth, including Calcium, Magnesium, Sulfur and other trace metals, but these are rarely limiting to the physiological functioning of plants. Agriculturists have long realized the positive effect of increasing macronutrient concentrations in the soil on plant performance, however relating nutrients to plant performance and identifying the mechanisms by which plants increase physiological activity has been one of the main endeavors of plant ecophysiology over the last several decades. From a more biological perspective, nutrients are the building blocks of life. Nucleic acids, and proteins are rich in Nitrogen and Phosphorus. Photosynthesis, at the leaf level, is the combination of a complex photo- and bio-chemical process (i.e., light and dark reactions). These reactions require a suite of specialized biological machinery, for example the thylakoids of the chloroplasts or the many specialized proteins and enzymes involved in photosynthesis, like the powerhouse protein of the Calvin-Benson cycle: Ribulose-1,5-bisphosphate carbolxylase/ oxygenase (RuBisCO). RuBisCO is probably the most abundant protein on earth; it is a very large protein with the molecular weight of 560,000 Dalton and is comprised of 15-50% chloroplast protein (Vapaavuori 1986), thus can be considered to be molecularly rich in N and, to a lesser degree, P. Variation in the amount of RuBisCO or other photosynthetically-functional proteins and enzymes contained in plant leaves affect their nutrient concentrations and photosynthetic performance. Accordingly, identifying how leaf tissue nutrient concentrations vary and correlate with measures of photosynthesis has led to a deeper understanding of the variability in plant form and function (e.g., Wright et al. 2004, Reich 2014). The typical way in which plant performance is measured is via carbon assimilation rates of leaves. Amazing instruments (e.g., the Li-6800 Portable Photosynthesis System, LiCOR, Lincoln, NE) can quantify the amount of carbon dioxide uptake by a leaf, by measuring its photosynthetic assimilation (A). By optimizing the abiotic environment of the plant and the immediate environment of leaf for photosynthesis, such as increasing light to a non-limiting level (>1000 μmols m-2 s-1) or controlling the temperature to be at the optimal 29°C (Slot and Winter 2017), one can measure the maximum rate of photosynthesis (Amax). Amax varies, with C3 plant usually having lower rates than C4 plants (Pons et al. 1998). For example, Amax for tropical tree species usually ranges between 10 and 20 μmols CO2 m-2 s-1 (see values reported by Slot and Winter 2017), where Amax for a corn plant (Zea mays) can be above 40 μmols CO2 m-2 s-1 (personal observation). This is due to differences in the biochemical pathways of photosynthesis between C3 and C4 plants; C4 plants have the ability to do carbon dioxide enrichment in bundle sheath cells. In C4 plants, CO2 moves through the typical light-dependent reactions in the mesophyll and is then transported via malate to the bundle sheath cells where the gas is concentration before being fixed again by RuBisCO in Calvin-Benson cycle. The release of CO2 (decarboxylation) of malate in the bundle sheath cells creates higher concentrations of CO2, meaning the Calvin-Benson cycle can operate more efficiently, thus increasing Amax (Schulze et al. 2005). The CO2 enrichment also creates lower rates of photorespiration in C4 plants. Accordingly, plants vary in the way in which they use CO2, but they also vary in the way in which they use other nutrients, for example N and P. Notably, leaf N content for corn can range from 17 to 45 mg g-1 in an agricultural setting (i.e., with application of fertilizer Schepers et al. 1992), which is within the global range of leaf nitrogen concentrations for all plants (Reich and Oleksyn 2004). Relationships between plant performance, leaf construction and leaf N First, leaf concentrations of nutrients, principally N and P, reflect their availability in the soil. In most terrestrial systems, plant growth is both N and P limited, and on average plants biologically require 10 times as much N as P, when growing optimally (Pons et al. 1998). Therefore, the N:P in leaf tissues is typically a good indicator of which nutrient is limiting, with N:P ratios <14 signifying N limitation, N:P ratios > 16 corresponding to P limitation and colimitation occurring at ratios between 14 and 16 (Aerts and Chapin III 1999). But moreover, leaf nutrient concentrations have been a surrogate trait for plant physiological performance (i.e., rates of photosynthesis). Species that photosynthesize at higher rates and grow faster, uptake nutrients from the soil at greater rates. A leaf economics spectrum ranges from cheaply-constructed leaves with short lifespans (i.e., little relative return on nutrient investment) to more-expensive, thicker leaves, with high leaf mass per area (LMA) and long lifespans (Wright et al. 2004). Along the leaf economics spectrum, higher N and P concentrations and faster A rates are associated with leaves of lower LMA (Wright et al. 2004, Reich 2014). Leaves with lower LMA tend to be associated with grasses, deciduous tree species or forbs, while evergreen trees tend to have higher LMA, lower A rates, and lower N and P leaf concentrations (Westoby and Wright 2006, Reich 2014). The amount of leaf N is correlated with the amount of leaf P (Niklas et al. 2005), both of which increase as LMA decreases (Wright et al. 2004). In other words, cheaply-constructed leaves with short lifespans have both higher concentrations of N and P, which covary. To understand the physiological tradeoffs in leaf performance (i.e., photosynthetic differences), we need to understand the economics of leaf construction. Leaf construction cost can be defined as the amount of carbon needed to produce a gram of leaf tissue relative to the associated “payback time” needed to recover that carbon via photosynthesis (Karagatzides and Ellison 2009). Leaves that are carbon-rich, tend to have high construction costs, but also tend to have long leaf lifespans. Typically, plants will further invest in secondary metabolites or latex into expensive leaves to guard against herbivory and ensure a longer-term return on nutrient investment. Ecologically, this is a k-strategy. Carbon-rich leaves tend to have lower maximum rates of photosynthesis (Wright et al. 2004), and species that produce expensive leaves tend to grow slower and have denser wood (Díaz et al. 2016) . Conversely, an r-strategist produces leaves with a fraction of the carbon investment per gram of leaf tissue, creating a thin, structurally-weaker leaf with a shorter lifespan. These leaves are also more susceptible to mechanical damage, pathogens or herbivory, however can be reproduced more readily by the plant, because the construction cost is low. They have higher maximum rates of photosynthesis, and are typically produced by heliophilic species with high growth and mortality rates (Wright et al. 2010). The basis of the relationships between leaf structure, leaf nitrogen concentrations and rates of photosynthesis arises from the fact that photosynthesis is a rate-limited process that uses both CO2 and macronutrients, mainly N & P. Most terrestrial ecosystems are either N or P limited, or N and P co-limited (Elser et al. 2007); so let us think of photosynthesis CO2 and nutrient-limited process. Assuming no light limitation and the fact that leaves want to maximize CO2 assimilation rates, we can decompose photosynthesis into two components: a portion of the process that is CO2-limited, and a portion of the process that is limited by the availability of reactants needed for the Calvin-Benson cycle, or nutrient-limited. The maximum rate of assimilation during the carboxylation reaction-limited portion of photosynthesis (Vcmax) and the maximum rate of assimilation when the photosynthetic machinery is limited by reactants (i.e., electron transport rate is constant) (Jmax), can give provide information on the relative strength of CO2 or nutrient limitation on leaf-level plant performance. Wullschleger (1993) compiled estimates of Vcmax and Jmax for 109 C3 plant species and found that Vcmax ranged from 6 (Picea abies; Norway Spruce) to 194 (Beta vulgaris; garden beet) μmols CO2 m-2 s-1 , and that Jmax ranged from 17 (again, Picea abies) to 372 (Mahastrtun rotundifolium; a desert annual) μmols CO2 m-2 s-1. Averages for Vcmax and Jmax across all 109 species were 64 and 139 μmols m-2 s-1, respectively, and when comparing values across plant lifeforms, Vcmax and Jmax were generally higher for herbaceous annals than for wood perennials. This confirms photosynthetic performance basis for the variation on plant leaf structural investment. Because plants use on average 10 times a much N as P, N concentrations have been more popular in investigating physiological relationships to leaf tissue nutrient concentrations. Leaf N does scale with chlorophyll, because proteins used in the photosynthetic dark reactions and those in the thylakoids are N-rich. In a brilliant study, John Evans (1989) showed that not all plants use N the same within a leaf, and that differences in N use vary based on the light environment and differences in leaf economics strategies among species. Specifically, shade-tolerant species on the resource-conservative end of the leaf-economics spectrum allocated a greater proportion of N to thylakoids in the Chloroplast, whereas resource-acquisitive species, with higher rates of photosynthesis allocated less to thylakoid proteins and more to Calvin cycle proteins and enzymes (e.g., RuBP carboxylase). Therefore, we can understand there to exist a tradeoff at the leaf-level on the relative return in nutrient investment in electron transport (J) relative to carboxylation (V) on assimilation rate (Maire et al. 2012, Walker et al. 2014). When carboxylation is limiting photosynthesis, an investment in maximizing Jmax has little effect on assimilation rate and leads to electron transport energy not used in photosynthesis. However, when light is limiting photosynthesis, a greater investment in Jmax relative to Vcmax maximizes photosynthetic rate, yet that greater investment is offset by the cost of energy dissipation when carboxylation is limiting, to avoid inhibition. Maire et al. (2012) employed this assumption about the relative investment of N in Jmax, its relationship to Vcmax and using species-specific parameter values for specific leaf area, created a leaf-level photosynthetic model that accounted for 93% of the total variance in leaf N across species and environmental conditions. In conclusion, leaf N varies because of physiological differences related to plant strategy. Plants with high leaf N have low LMA and high rates of photosynthesis. The biological explanation for the higher concentrations of leaf N in these species, is in short, that they need it do the work of assimilating CO2 at high rates. Works cited: Aerts, R., and F. S. Chapin III. 1999. The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Pages 1-67 Advances in Ecological Research. Elsevier. Díaz, S., J. Kattge, J. H. Cornelissen, I. J. Wright, S. Lavorel, S. Dray, B. Reu, M. Kleyer, C. Wirth, and I. C. Prentice. 2016. The global spectrum of plant form and function. Nature 529:167. Elser, J. J., M. E. Bracken, E. E. Cleland, D. S. Gruner, W. S. Harpole, H. Hillebrand, J. T. Ngai, E. W. Seabloom, J. B. Shurin, and J. E. Smith. 2007. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters 10:1135-1142. Evans, J. R. 1989. Photosynthesis and nitrogen relationships in leaves of C 3 plants. Oecologia 78:9-19. Karagatzides, J. D., and A. M. Ellison. 2009. Construction costs, payback times, and the leaf economics of carnivorous plants. American Journal of Botany 96:1612-1619. Maire, V., P. Martre, J. Kattge, F. Gastal, G. Esser, S. Fontaine, and J.-F. Soussana. 2012. The coordination of leaf photosynthesis links C and N fluxes in C3 plant species. PloS One 7:e38345. Niklas, K. J., T. Owens, P. B. Reich, and E. D. Cobb. 2005. Nitrogen/phosphorus leaf stoichiometry and the scaling of plant growth. Ecology Letters 8:636-642. Pons, T., H. Lambers, and F. Chapin III. 1998. Plant Physiological Ecology. 2nd Edition edition. Springer-Verlag, New York. Reich, P. B. 2014. The world‐wide ‘fast–slow’ plant economics spectrum: a traits manifesto. Journal of Ecology 102:275-301. Reich, P. B., and J. Oleksyn. 2004. Global patterns of plant leaf N and P in relation to temperature and latitude. Proceedings of the National Academy of Sciences 101:11001-11006. Schepers, J., D. Francis, M. Vigil, and F. Below. 1992. Comparison of corn leaf nitrogen concentration and chlorophyll meter readings. Communications in Soil Science and Plant Analysis 23:2173-2187. Schulze, E., E. Beck, and K. Müller-Hohenstein. 2005. Plant Ecology. Springer, Berlin. Slot, M., and K. Winter. 2017. In situ temperature response of photosynthesis of 42 tree and liana species in the canopy of two Panamanian lowland tropical forests with contrasting rainfall regimes. New Phytologist 214:1103-1117. Vapaavuori, E. 1986. Correlation of Activity and Amount of Ribulose 1, 5-bis phosphate Carboxylase with Chloroplast Stroma Crystals in Water-Stressed Willow Leaves. Journal of Experimental Botany 37:89-98. Walker, A. P., A. P. Beckerman, L. Gu, J. Kattge, L. A. Cernusak, T. F. Domingues, J. C. Scales, G. Wohlfahrt, S. D. Wullschleger, and F. I. Woodward. 2014. The relationship of leaf photosynthetic traits–Vcmax and Jmax–to leaf nitrogen, leaf phosphorus, and specific leaf area: a meta‐analysis and modeling study. Ecology and Evolution 4:3218-3235. Westoby, M., and I. J. Wright. 2006. Land-plant ecology on the basis of functional traits. Trends in Ecology & Evolution 21:261-268. Wright, I. J., P. B. Reich, M. Westoby, D. D. Ackerly, Z. Baruch, F. Bongers, J. Cavender-Bares, T. Chapin, J. H. Cornelissen, and M. Diemer. 2004. The worldwide leaf economics spectrum. Nature 428:821. Wright, S. J., K. Kitajima, N. J. Kraft, P. B. Reich, I. J. Wright, D. E. Bunker, R. Condit, J. W. Dalling, S. J. Davies, and S. Diaz. 2010. Functional traits and the growth–mortality trade‐off in tropical trees. Ecology 91:3664-3674. Wullschleger, S. D. 1993. Biochemical limitations to carbon assimilation in C3 plants—a retrospective analysis of the A/Ci curves from 109 species. Journal of Experimental Botany 44:907-920.
1 Comment
|
AuthorJames "Aaron" Hogan is an ecologist interested in plant biodiversity, forests and global change. Archives
November 2021
Categories |